Abstract

Introduction: With a prevalence of 1 in 5000 persons, hereditary hemorrhagic telangiectasia (HHT) is the second-most common hereditary bleeding disorder in the world. As HHT is a bleeding disorder with a concomitant elevated thrombotic risk, antithrombotic therapy (anticoagulation and antiplatelet therapy) is frequently needed, but data describing its use is extremely limited. Antithrombotic therapy recommendations in the recently published Second International HHT Guidelines are based on this very limited data (largest prior cohort study including 28 patients) or expert opinion alone.
Methods: We evaluated outcomes in a 5-hospital observational cohort study of adults with HHT receiving antithrombotic therapy over a 26-year period. Patients were identified via electronic database query, and eligibility was confirmed and data collection performed via manual chart review. Multivariable logistic modeling was used to assess for predictors of premature antithrombotic therapy discontinuation and hematologic characteristics and healthcare utilization before and after initiation of antithrombotic therapy were evaluated using paired t-tests (continuous data) and chi-square tests (categorical data).
Results:Patients and Episodes: 119 HHT patients with 187 discrete antithrombotic therapy episodes were included. Median (range) age was 70 (27-92) years and 69 patients (58%) were female. Most common antithrombotic indications were venous thromboembolism (61 episodes), coronary artery disease (29 episodes), and atrial fibrillation (29 episodes). Most common antithrombotic agents used (by number of episodes) were aspirin (57), warfarin (35), low molecular weight heparin (26), apixaban (15), rivaroxaban (8), and multiple simultaneous medications (41).
Tolerability and Effectiveness Overall and by Antithrombotic Therapy Class: 59 patients (50%) prematurely discontinued and/or dose-reduced therapy (including 52 patients [44%] who discontinued) due to worsening bleeding. Initiation at reduced dose-intensity had a similar premature discontinuation rate (49%) as initiation at standard dose-intensity (43%). Difficulty receiving indicated therapy may have resulted in increased thromboembolic recurrence (20 patients, 17%). Rates of dose-reduction and/or premature discontinuation were similar regardless of anticoagulant class (warfarin, 46%; heparin-based, 48%; direct oral anticoagulant [DOAC], 44%) or with multiple simultaneous agents (44%) but slightly lower with single-agent antiplatelet therapy (37%).
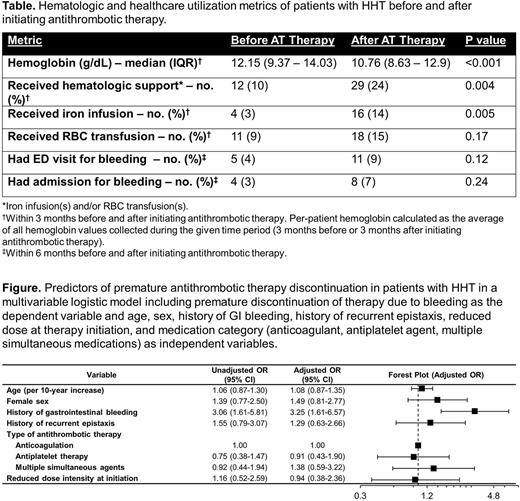
Predictors of Therapy Discontinuation: In a multivariable logistic model, a history of GI bleeding was associated with 3.25-fold odds of discontinuation (P=0.001, Figure).
Adverse Events Associated with Antithrombotic Therapy: Hemoglobin was significantly lower (10.8 g/dL vs 12.2 g/dL, P<0.001), and need for intravenous iron and RBC transfusion significantly higher (29 patients vs 12 patients, P=0.004), in the 3 months after antithrombotic therapy initiation versus the 3 months before (Table); number of patients requiring ED visits and/or hospital admissions due to bleeding also increased. 75 patients (63%) developed worsened epistaxis (of whom 33% dose-reduced or discontinued antithrombotic therapy due to epistaxis) and 44 patients (37%) developed newly-diagnosed or worsened gastrointestinal bleeding (of whom 59%, dose-reduced or discontinued antithrombotic therapy due to GI bleeding).
Conclusions: Successful administration of antithrombotic therapy is challenging in HHT, resulting in objectively higher morbidity and healthcare utilization from worsened bleeding. Discontinuation rates approached 50% regardless of dose-intensity at initiation or type of antithrombotic agent used and were higher in patients with a history of gastrointestinal bleeding. These findings, from the largest and most comprehensive evaluation of antithrombotic therapy in HHT to date, challenge at least two recommendations made based on limited prior data or expert opinion alone in the Second International HHT Guidelines: the recommendation for use of warfarin or heparin-based therapy over DOACs and the avoidance of multiple simultaneous agents even when otherwise indicated due to concern for higher bleeding risk.
Disclosures
Rodriguez-Lopez:Bayer: Consultancy, Research Funding; United Therapeutics: Consultancy, Research Funding; Altavant Sciences: Consultancy; Merck: Research Funding. Holbrook:Lyra Therapeutics: Consultancy. Al-Samkari:Rigel: Consultancy; Forma: Consultancy; Agios: Consultancy, Research Funding; argenx: Consultancy; Sobi: Consultancy, Research Funding; Amgen: Research Funding; Novartis: Consultancy; Moderna: Consultancy; Dova: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal